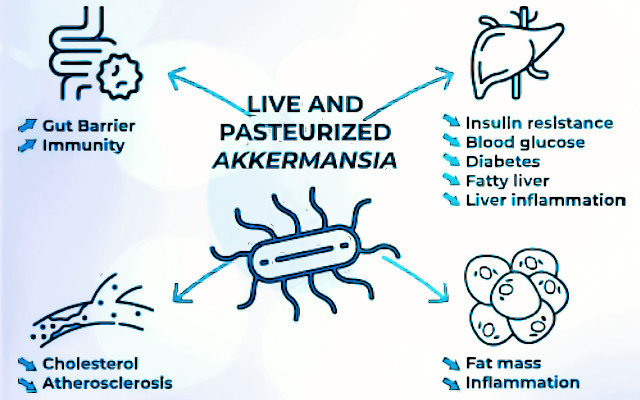
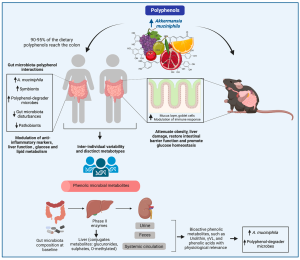
The intestinal microbiota is honored as the key regulator of host homeostasis. The compositional imbalance of microbial communities commonly causes metabolic disorders and exacerbates disease progression. Akkermansia muciniphila (Akk) is a strict anaerobe Gram-negative bacterium which first isolated from human feces in 2004. AKK uses mucin as its sole carbon, nitrogen, and energy source. Akkermansia muciniphila is one of the most abundant single species in the human intestinal microbiota. Since its discovery in 2004, the role of Akk in human metabolic health and disease therapy has been widely studied and therefore it has been called “next-generation beneficial microbes” and“one of the most promising probiotics”. Up to now, many papers associated with Akermansia muciniphila have been published, with diabetes and obesity as the factors under the greatest focus, and cancer as the hot topic of recent research. In the last decade, it was uncovered that Akermansia muciniphila can prevent and ameliorate metabolic syndrome, obesity, diabetes, aging, inflammation, neurodegenerative diseases, and negative effects of cancer therapy. Although it is comprehensively elaborated on the critical functions of Akermansia muciniphila in the metabolism of human health and nutrient utilization, the exhaustive signaling molecular mechanism of Akermansia muciniphila interacting with the host is still not completely understood. Yet, it is worth noting that several studies found that elevated Akk abundance as a paradigm for “next-generation beneficial microorganisms” is positively associated with aggravated host disease progression.
The Cultivation Characteristics of Akkermansia muciniphila
Akk is strictly anaerobic and mainly grown in mucin medium but also can grow on a limited number of sugars including N-acetylgalactosamine, N-acetylglucosamine or a synthetic medium constituted of glucose, peptone, and threonine that has been confirmed to be safe for human administration.
Glucosamine-6-phosphate (GlcN6P) exists in mucin is also necessary for Akk growth, and promoting adaptation to the mucosal niche. Nowadays, the mucin medium is widely and mainly used
to cultivate Akk, but the risk of animal-derived mucins is incompatible with human administration. The cultivation and growth of Akk need strictly anaerobic conditions, which make its mass production more challenging, and it still needs further investigations for improving its cultivation method and safety for human oral administration.
Akkermansia muciniphila in Preventing and Ameliorating Obesity and Metabolic Disorders
Obesity and type2 diabetes are associated with low-grade inflammation and specific changes in gut microbiota composition such as Akk prevalence. In addition to the genome of Akk, the membrane proteins and extracellular vesicles (EVs) of Akk have attracted more attention in the prevention and treatment of obesity and type 2 diabetes. Individuals with obesity have a significantly higher
abundance of Firmicutes, whereas the abundance of Akk is significantly decreased in individuals with obesity and diabetes which is inversely associated with body fat mass and glucose intolerance. The abundance of Akk is strongly correlated with body mass index (BMI) and antidiabetic drug usage. Akk alleviates -induced metabolic disorders, including fat mass gain, metabolic endotoxemia, adipose tissue inflammation, and insulin resistance, as well as increases the intestinal levels of endocannabinoids that regulate inflammation, gut barrier and gut peptide secretion. In high fat diet -induced diabetic mice, EV treatment can improve intestinal barrier integrity by increasing tight junction (TJ) protein expression in an AMP-activated protein kinase -dependent manner. Akk also ameliorates chronic low-grade inflammation by decreasing plasma levels of lipopolysaccharide (LPS)-binding protein (LBP) and leptin and inactivating LPS/LBP downstream signaling mediated.
Conclusions and Future strategies
Critical contributions of Akermansia muciniphila toward metabolic health have begun to be elucidated. In particular, more recent studies is revealing how the effects of Akk extend beyond the GI tract, especially for the so-called gut–brain, gut–liver, gut–bone, gut–heart, gut–adipose and gut–muscle tissue axes, and cancer therapy. To date, the primary focus has been on the use of Akk to ameliorate diabetes, obesity, metabolic diseases, neurodegenerative diseases, inflammation, aging, and the negative effects of cancer therapy. The crucial knowledge gaps remain in this area, specifically on how Akk modulates lipid and glucose metabolism, brain metabolism, and the immune response. However, negative effects of Akk and the safe dose are comparatively less well understood.
Prepared by: Dr. Nazila Kassaian
References
- Jian H, Liu Y, Wang X, Dong X, Zou X. Akkermansia muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut–Liver–Brain Axes?. International Journal of Molecular Sciences. 2023 Jan; 24(4):3900.
- Yoon H.S, Cho C.H, Yun M.S, Jang S.J, You H.J, Kim J.-H, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–۵۷۳.
- Chelakkot C, Choi Y, Kim D.-K, Park H.T, Ghim, J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med.2018, 50, e450.
- Keshavarz Azizi Raftar S, Ashrafian F, Yadegar A, Lari A, Moradi H.R, et al. The Protective Effects of Live and Pasteurized Akkermansia muciniphila and Its Extracellular Vesicles against HFD/CCl4-Induced Liver Injury. Microbiol. Spectr. 2021, 9, e0048421.