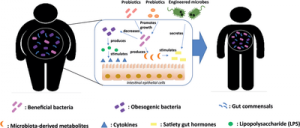
In the gut, the microbiota mostly comprised bacteria, but it also harbors archaea, viruses, protozoans, and fungi. The composition of the gut microbiota is unique to each individual in that the gut microbiota of two given individuals consistently show differences in their composition. Nonetheless, it is also highly dynamic and evolves throughout life under the influence of a wide diversity of genetic, environmental, medical, and dietary determinants.
The microbiota and intensive care patients
In the intensive care setting, the gut microbiota of patients is submitted to various stresses including antibiotic exposure, modification of gastrointestinal transit, artificial nutrition or sepsis which may lead to a dysbiosis during hospitalization. Indeed, the gut microbiota in critically ill patients appears to be different from that of healthy subjects, demonstrating markedly lower richness and diversity, and the near replacement of commensal genera by opportunistic pathogens. Recent evidence has shown that dysbiosis in ICU patients might have consequences on survival, stressing that dysbiosis could be considered as an authentic, organ-failure-affecting prognosis along with renal, cardiac, or respiratory failures.
Dysbiosis alteration and patients’ management
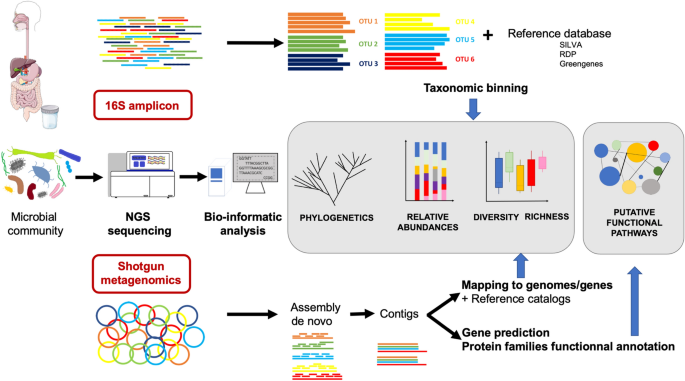
The gut microbiota mainly includes difficult-to-cultivate anaerobic bacteria, hence knowledge about its composition has significantly arisen from culture-independent methods based on next-generation sequencing (NGS) such as 16S profiling and shotgun metagenomics.
Correcting the microbiota disturbances to avoid their consequences is now possible. Fecal microbiota transplantation is recommended in recurrent C. difficile infections and microbiota-protecting treatments such as antibiotic inactivators are currently being developed. The growing interest in the microbiota and microbiota-associated therapies suggests that the control of the dysbiosis could be a key factor in the management of critically ill patients.
The gut microbiota is also the main reservoir for multidrug-resistant bacteria organisms (MDRO). Initially kept at low intestinal concentrations as a consequence of the barrier effect exerted by commensal anaerobic bacteria, they may bloom after antibiotic exposure and increase the risk their involvement in further infections.
prepared by: Nazila Kassaian
Reference
Szychowiak P, Villageois-Tran K, Patrier J, Timsit JF, Ruppé É. The role of the microbiota in the management of intensive care patients. Ann Intensive Care. 2022 Jan 5;12(1):3. doi: 10.1186/s13613-021-00976-5. PMID: 34985651; PMCID: PMC8728486.