The gut microbiome has been identified as a potential factor in weight regulation. Thirty-nine percent of the adults are overweight (25–۲۹.۹ kg/m2) and 13% are obese (BMI≥۳۰kg/m2) worldwide. Recently, it has been found that obesity may affect brain function and structure, as it is associated with impaired cognition and alterations in gray matter (GM) and white matter (WM). Moreover, a higher BMI and waist-to-hip ratio (WHR) have been associated with lower fractional anisotropy (FA) values. Moreover, it is proposed that obesity increases the risk of developing dementia later in life by 60–۹۰%, versus healthy weight individuals. A growing of evidence reveals that obesity is related with alterations in neuroendocrine production and secretion, including ghrelin, insulin, GLP-1 and PYY.
Bariatric surgery for obesity treatment
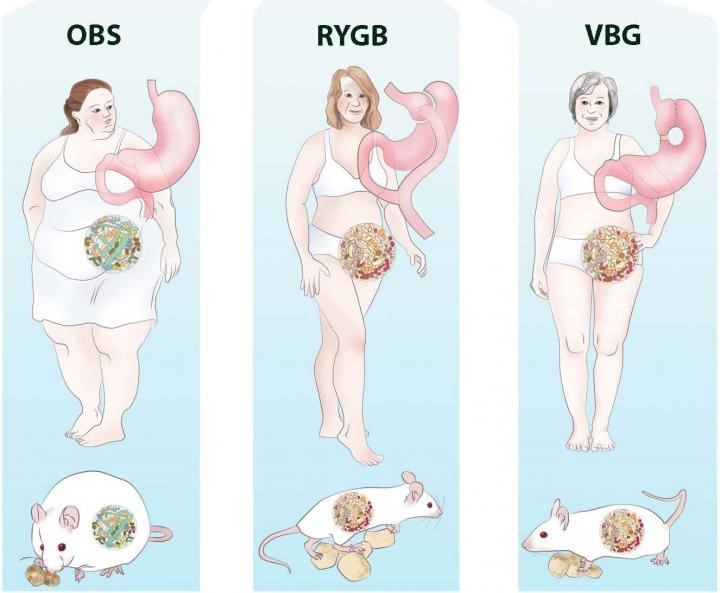
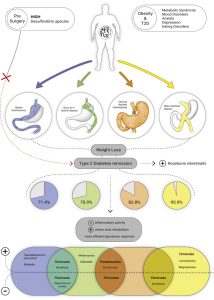
Bariatric surgery is an effective treatment for obesity leading to rapid and sustainable weight loss. Bariatric surgery decreases body weight not only due to physical effects such as reduced food intake and malabsorption but also due to the various neuroendocrine changes which affect energy homeostasis and hunger/satiety. Moreover, Bariatric surgery might improve the gut microbiota diversity and restore white adipose tissue function, which can improve obesity-related immunological and cognitive impairments. The gut-brain axis consists of a bidirectional communication system, connected through the vague nerve, spinal fibers and sympathetic and parasympathetic fibers which are directly innervating the gastrointestinal tract. These elements communicate through endocrine messengers, neuro-immune mediators and neuroactive metabolites.
Conclusion
To summarize, Bariatric surgery is a good procedure to treat obesity and its related pathologies, however long-term effects remain unsolved. Future research should focus on the long-term effects of Bariatric surgery, to be able to investigate the neuroendocrine, microbiota and white adipose tissue changes and to potentially determine the new “normal” after homeostatic adjustments. Various studies have focused on neuroendocrine alterations already after six months. Six months post-surgery patients lose weight rapidly and generally still follow their post-operative diet. Therefore, the observed effects 6 months post-surgery might differ at longer follow-ups, when patients achieve a stable weight, or regain weight.
References
- de Wit DF, Hanssen NM, Wortelboer K, Herrema H, Rampanelli E, Nieuwdorp M. Evidence for the contribution of the gut microbiome to obesity and its reversal. Science Translational Medicine. 2023 Nov 22; 15(723):eadg2773.
- Custers E, Franco A, Kiliaan AJ. Bariatric Surgery and Gut-Brain-Axis Driven Alterations in Cognition and Inflammation. Journal of Inflammation Research. 2023 Dec 31:5495-514.
Provided by: Dr. Nazila Kassaian