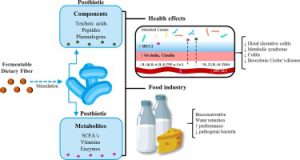
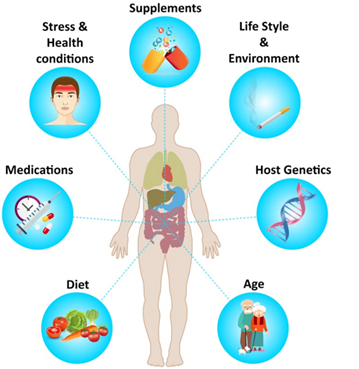
Over 10 trillion microbial cells in the human GI produce specific metabolites and bioactive compounds that trigger the host’s immunological and metabolic pathways. Microbial symbiosis and stable human-intestinal microbiota communities that promote health and are resistant to disruption require this homeostatic symbiosis between the host and the microbiota. Postbiotics, a novel biotic, are inanimate microbes and their components that benefit the host. Microbial activity produces postbiotics, which benefit the host’s gut health. During lysis, bacteria release enzymes, peptides, teichoic acids, peptidoglycan-derived muropeptides, polysaccharides, cell surface proteins, and organic acids.
Postbiotics are bioactive compounds created after fermentation in the matrix that can alleviate food allergies and enhance immunological tolerance, especially in young children and infants. Structure, elemental makeup, proteins, vitamins, lipids, organic acids, and complex compounds are used to categorize them. Postbiotics have immunological effects that boost mucin formation and promote claudin synthesis. They show promise for the early detection and effective management of digestive disorders in children. The optimal parent cell strains, doses, and reasonably priced postbiotics will require further study. High pressure, ultraviolet light, formalin inactivation, thermal treatments, ionizing radiation, and sonication can increase food nutrition, shelf life, and health.
The Impacts of Postbiotics in Human Health
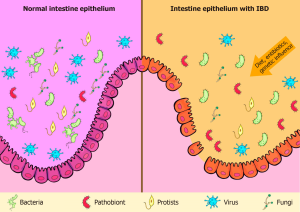
Postbiotics are immunomodulatory, antihypertensive, anti-inflammatory, antiproliferative, hypocholesterolemia, anti-obesogenic, and antioxidant. Probiotics like teichoic acid, indole, lipopolysaccharide, muramyl dipeptide, and lactospin make them. They boost intestinal health-promoting Lactobacillus and Bifidobacterium bacteria. Postbiotics lower blood sugar and improve insulin function in obese persons. They have anti-inflammatory, anti-hypertensive, immunomodulation, hypocholesterolemia, anti-proliferative, anti-obesogenic, and antioxidant properties. Postbiotic advantages depend on the microbe or bacteria utilized.
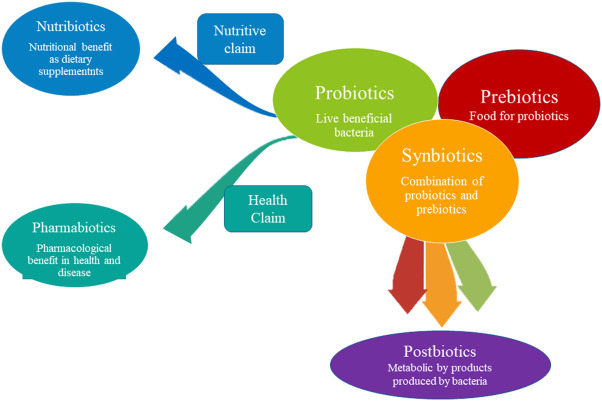
Postbiotics get preference over probiotics
Postbiotics improve gut microbiota health and have antibacterial, immune-modulating, and anti-inflammatory properties. They last less than probiotics and are easier to travel, store, and maintain. Postbiotics are safer, faster to produce, and protect against virulence factors and antibiotic resistance genes. They improve immune system maturation and treat allergies. Postbiotics have clear chemical structures, long shelf lives, and safe doses. They imitate probiotic health advantages without live germs, making them a safer alternative to live probiotics.
Classification of postbiotics
Enzyme synthesis, carbohydrate fermentation, and vitamin and peptide synthesis are postbiotics. Proteins, organic acids, lipids, carbohydrates, vitamins, and complex compounds comprise postbiotics. SCFAs, plasminogen, teichoic acids, vitamins, peptides, and enzymes are postbiotics.
Postbiotic’s extraction
Metabolomics quantifies micro molecules in complex biological systems, making it perfect for postbiotic detection. Centrifugation and ultrafiltration are the most common ways to extract postbiotics. Proteolytic microorganisms start lab fermentations to maintain pH and optimize postbiotic release.
References
Provided by: Dr. Babak Tamizifar