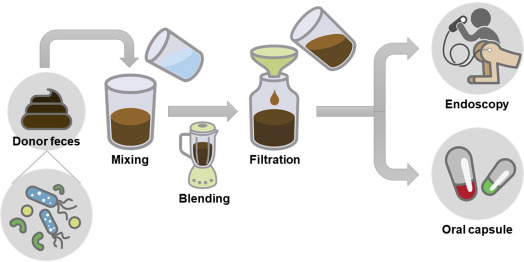
Nowadays, some strategies like probiotics, prebiotics, post biotics, synbiotics or fecal microbiota transplantation (FMT) rely on adding individual, several, or a whole consortium of living microbial organisms to exclude disease-causing microbes and provide health-promoting benefits. Moreover, bacteriocins and bacteriophages present another potential strategies to remove specific pathogens associated with the onset of a particular diseases; however, their exploration as therapeutics in humans is still in its infancy.
Gut-microbiota targeted therapeutics in IBD
The therapeutic potential of these agents in inflammatory bowel disease (IBD) comprising ulcerative colitis (UC) and Crohn’s disease (CD), has been evaluated in a meta-analysis of 32 randomized controlled trials (RCTs). The authors found that these therapeutics considerably increased the number of beneficial intestinal bacteria (particularly Bifidobacterium), induced or maintained IBD remission and lowered UC disease activity index whilst not affecting IBD recurrence. Subgroup analyses showed that combining probiotics and prebiotics with conventional therapies was more effective in reducing these parameters than traditional treatments alone, while synbiotic treatment seemed to be more effective than prebiotics and probiotics alone. Additionally, the study suggested that probiotics containing Bifidobacterium, Lactobacillus, or more than one bacterial strain were more effective as IBD therapeutics and proposed doses from 10۱۰ to 10۱۲ colony forming units (CFU)/day as reference dose. The severity of inflammation and disease activity has also been proposed to influence the effectiveness of microbiota-targeted therapeutics in IBD.
Moreover, the data could suggest that the microbiota-modulatory efficacy of prebiotics decreases going from healthy, at-risk subjects to those with inactive and active IBD, indicating their potential in IBD primary prevention and treatment in a less inflamed gut.
Gut-microbiota targeted therapeutics in diarrhea
The effect of probiotics on chronic diarrhea, associated with different intestinal disorders like irritable bowel syndrome (IBS) and functional diarrhea, was evaluated. Yang et al have shown that intake of Lactiplantibacillus plantarum CCFM1143 for 4 weeks can be effective in managing chronic diarrhea symptoms in patients compared to placebo (maltodextrin). Moreover, it has been demonstrated that prebiotic consumption decreases the abundance of Bacteroides and Eggerthella, increases the abundance of beneficial species like Akkermansia, Terrisporobacter, and Anaerostipes, and stimulates acetic and propionic acid production. These data suggesting the potency of this probiotic strain and prebiotic can improve the microbiota imbalance and clinical symptoms in functional bowel disorders.
Gut-microbiota targeted therapeutics in Helicobacter pylori infection
The therapeutic potential of probiotics alone or in combination with standard treatments has also been evaluated in Helicobacter pylori infection. One study showed that consumption of a probiotic drink containing fermented milk with Lacticaseibacillus paracasei CNCM I-1518 and I-3689, L. rhamnosus CNCM I-3690, and four yogurt strains for 28 days induced faster gut microbiota recovery after H. pylori eradication, reducing the abundance of potentially pathogenic bacteria (e.g., Escherichia-Shigella and Klebsiella) and increasing fecal SCFA generation compared to the control drink. Another study evaluated the therapeutic effects of a probiotic including Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus, provided alone or in combination with quadruple eradication therapy (PPI, bismuth, and two antibiotics) for 2 weeks, on gastric microbiota recovery in H. pylori-infected individuals. Results showed that 2 months after treatment, the quadruple therapy did not restore gastric microbiota of H. pylori-positive subjects to an uninfected state; however, adjuvant probiotic therapy contributed to its recovery by improving microbial diversity, reducing the abundance of potentially harmful bacteria (e.g., Fusobacterium, Campylobacter and Proteobacteria) and increasing the beneficial bacteria (e.g., Lachnospiraceae, Ruminococcaceae, Eubacterium ventriosum). By contrast, probiotic monotherapy was ineffective in H. pylori abolition and failed to restore gastric microbiota, with observed alterations in microbiota structure, increased putative pathogenic bacteria, and no induction of beneficial bacteria.
written by: Dr.Nazila Kassaian