This study discusses the results, limitations, and future considerations involved in conducting the first fecal microbiota transplant (FMT) trial for ulcerative colitis (UC) in children. A randomized controlled trial was conducted in 25 pediatric patients with ulcerative colitis (13 in the FMT and 12 in the placebo arms). The composite clinical endpoint of this study was reached in the majority (92%) of the recipients assigned to FMT compared with 50% assigned to placebo at week 6. This trial highlighted important lessons associated with FMT trials in children.
Indeed, this study offers the first trial evidence that FMT may have a role in pediatric UC management.
The Study on Fecal Microbiota Transplant in Inflammatory Bowel Disease
While drug development continues to focus on modifying dysregulated intestinal immune pathways, targeting enteric microbiota has become increasingly attractive. Four randomized controlled trials of fecal microbiota transplant in UC have been conducted since 2015. Three demonstrated clinical and endoscopic remission in adult patients with active UC, and a 2017 systematic review reported a pooled rate of clinical and endoscopic remission of 27.9%, with a number needed to treat of 5.
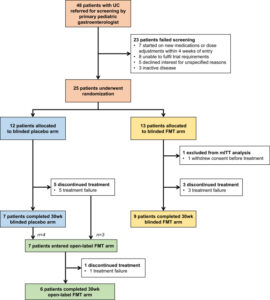
Figure ۱. Flow of screened, and randomized patients in the trial. FMT, fecal microbiota transplant; mITT, modified intention to treat; UC, ulcerative colitis.
Results of the First Pilot Randomized Controlled Trial of Fecal Microbiota Transplant in pediatric Ulcerative Colitis
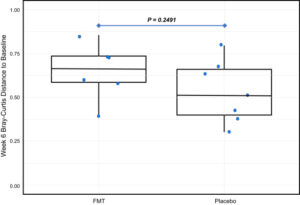
In this study, during a 36-month, 48 patients, aged 4 to 17 years, with active UC were referred by the patients’ primary pediatric gastroenterologists for screening across 3 pediatric IBD centers in Canada. Of these, 23 patients were excluded because of medication changes, unable to fulfill trial requirements FMT treatments, unspecified reasons, and remission by trial commencement. Ultimately, 52.1% (n= 25) of referred patients entered the trial. Patients were randomized to FMT (n = 13) or placebo (n = 12) arms. The composite clinical endpoint (improvement in pediatric UC activity index, C-reactive protein, or fecal calprotectin) in 92% (11 of 12) assigned to FMT vs 50% (6 of 12) assigned to placebo at week 6 was seen (risk ratio, 1.8; 95% CI, 1.1–۳.۷). At 12 months, 75% (9 of 12) had maintained clinical response. β-Diversity trended higher from baseline to week 6 in FMT vs placebo arms.
Supplementary Figure ۱. Change in microbiota composition between patients receiving fecal microbiota transplant vs placebo from baseline to week 6. The horizontal line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively; the vertical lines mark minimum and maximum of all the data, and the circles indicate individual data points.