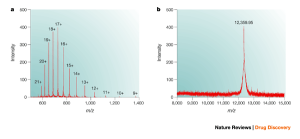
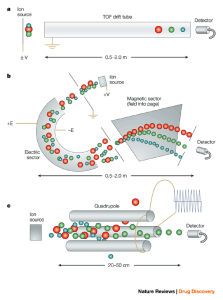
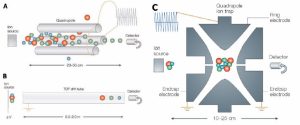
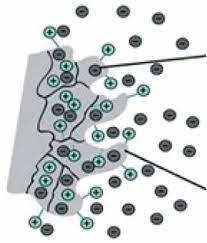
Electrophoresis or cataphoresis is the same as chromatography in which charged molecules (such as nucleic acids and proteins) are placed in an electric field and separated based on size. In the diagnosis of a broad range of diseases, like gastrointestinal disorders, immunodeficiency, and inflammatory diseases, the results of serum protein electrophoresis are appropriate.
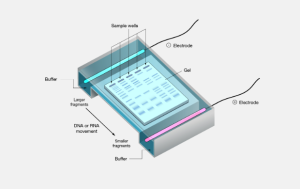
Figure 1. Schematic view of electrophoresis
Some electrophoresis types used in clinic
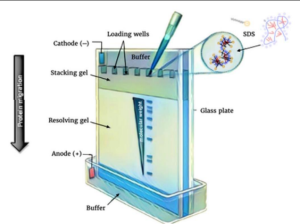
۱- Polyacrylamide gel electrophoresis (PAGE) → is used for the quantification, comparison, and description of proteins, peptides and nucleic acids with low molecular weight in serum or urine.
Figure 2. Polyacrylamide Gel Electrophoresis (PAGE)
۲- Agarose gel electrophoresis (AE) → is used for detection of protein abnormalities in various biological fluids, analysis of serum proteins, hemoglobin variants, lipoprotein fractions, and separation of DNA or RNA fragments of different lengths.
Figure 3. Agarose Gel Electrophoresis
۳- pulsed-field gel electrophoresis (PFGE) → is used for separation of large DNA molecules belonging to many living species including bacteria, viruses and mammals.
Figure 4. Pulsed-Field Gel Electrophoresis
۴- Isoelectric focusing (IEF) → is often used to demonstrate batch consistency as part of the quality control testing of therapeutic biological products.
Figure 5. Isoelectric focusing
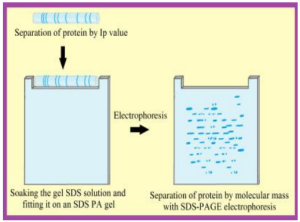
۵- Two dimensional electrophoresis (2-DE) → is a powerful tool for separating complex mixtures of proteins from tissues, cells, or other biological samples.
Figure 6. Two-Dimensional (2DE) gel electrophoresis
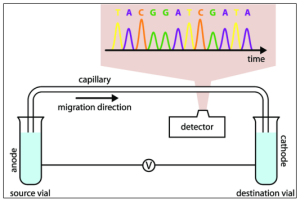
۶- Capillary electrophoresis (CE) → is used for pharmaceutical discoveries (especially in the case of anti-cancer drugs) and genetic analyses.
Figure 7. Capillary Electrophoresis
۷- Microchip electrophoresis (ME) → is widely used in mutation analysis, genotyping, immunological tests and small molecule detection and has been very successful in diagnosing of diabetes, pancreatic disorder, cancer, immune and genetic disorders, cardiovascular and infectious diseases.
Figure 8. Microchip Electrophoresis
۸- Immune electrophoresis → is widely used to identify protein changes in inflammation, liver and kidney diseases.
۹- Immunofixation electrophoresis → is a golden standard method for diagnosis and follow-up of multiple myeloma and paraproteinemia, genetic studies, and clinical trials.
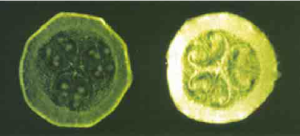
Figure 9. Immunofixation electrophoresis (IFE) on serum from 4
patients
۱۰- Hemoglobin electrophoresis → Thalassemia, and abnormal hemoglobins variants, can be identified.
۱۱- Automatic Electrophoresis System → can be used for the completion of the Human Genome Project, the detection of conformation polymorphism in genetic samples, and protein analysis with High efficiency.
References
- Fesmire, J.D., (2019). A Brief Review of Other Notable Electrophoretic Methods. In Electrophoretic Separation of Proteins (pp:495-499). Humana Press, New York, NY.
- Gowenlock, A.H., McMurray, J.R., and McLauchlan, D.M., (1987). Separative Procedures, Electrophoresis. In: Varley’s Practical Clinical Biochemistry. Heinman Medical Books, London. 69-81.
- Judd, R.C., (1996). SDS-polyacrylamide Gel Electrophoresis of Peptides. In The Protein Protocols Handbook (pp:101-107).
- Hames, B.D., (1998). Gel Electrophoresis of Proteins A Practical Approach Third Edition. Edited by School of Biochemistry and Molecular Biology.
- Giot, J.F., (2010). Agarose gel Electorphoresis–applications in Clinical Chemistry. JMB., 29:9–۱۴.
- Nedelcu, S. and Watson, J.H.P., (2004). Size Separation of DNA Molecules by Pulsed Electric Field Dielectrophoresis. J. Phys. D: Appl. Phys., 37:2197–۲۲۰۴.
- McEllistrem, M.C., Stout, J.E., and Harrison, L.H. (2000). Simplifield Protocol for Pulsed-Field Gel Electrophoresis Analysis of Streptococcus pneumoniae. Journal of Clinical Microbiology., 38:351-353.
- Magdeldin, S., Enany, S., Yoshida, Y., Xu, B., Zhang, Y., Zureena, Z., and Yamamoto, T., (2014). Basics and Recent Advances of Two Dimensional-Polyacrylamide Gel Electrophoresis. Clinical proteomics, 11(1):16.
- Tristezza, M., Gerardi, C., Logrieco, A., and Grieco, F., (2009). An Optimized Protocol for the production of interdelta markers in Saccharomyces Cerevisiae By Using Capillary Electrophoresis. Journal of Microbiological Methods., 78:286–۹۱.
- Wuethrich, A. and Quirino, J.P., (2019). Decade of Microchip Electrophoresis for Clinical Diagnostics – A review of 2008-2017. Analytica Chimica Acta., 1045:42-66.
- Michels, D.A., Hu, S., Schoenherr, R.M., Eggertson, M.J., and Dovichi, N.J., (2002). Fully Automated Two-dimensional Capillary Electrophoresis for High Sensitivity Protein Analysis. Mol Cell Proteomics., 1:69-74.
prepared by: Parvin Zarei