The mutual relationships and metabolic activities among microorganisms have a significant impact on modulate hormone levels, especially estrogens in women. The gut microbiota mainly controls estrogen levels through the secretion of β-glucuronidase, which is encoded by several microbiome genera, including Bacteroides, Bifidobacterium, Escherichia, and Lactobacillus. β-glucuronidase enzyme converts conjugated estrogens to deconjugated forms in the gastrointestinal tract. These deconjugated and unbound “active” estrogens enter the bloodstream and subsequently act on estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ), eliciting downstream activation of intracellular signaling cascades, gene transcription, and epigenetic effects. A decrease in β-glucuronidase activity due to an imbalance in the GM community (dysbiosis), there is less estrogen deconjugation, resulting in lower circulating estrogen levels. Conversely, increased β-glucuronidase activity can increase estrogen levels. Thus, maintaining optimal β-glucuronidase activity is critical for regulating estrogen levels in females. Estrogens contribute to epithelial proliferation throughout the female reproductive system and have been shown to drive proliferative diseases such as endometriosis and polycystic ovary syndrome (PCOS).
There is evidence of lower SCFAs concentrations in fecal samples from PCOS patients. Indeed, probiotics’ supplementation promoted the growth of Faecalibacterium prausnitzii, Bifidobacterium, and Akkermansia, which are SCFA-producing bacteria, and can lead to an increase in intestinal SCFAs. In turns, SCFAs bind to their receptors on enter endocrine cells and directly stimulate the release of gut–brain mediators that can influence sex hormone secretion by the pituitary gland and hypothalamus via the gut–brain axis.
Vaginal Microbiota Transplantation and Gynecological Disorders
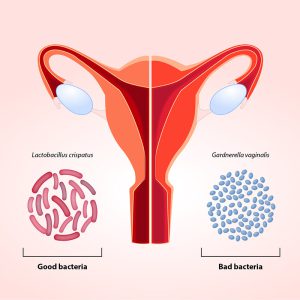
Gynecological disorders that have been explored in relation to Vaginal Microbiota Transplantation (VMT) include bacterial vaginosis, vulvovaginal candidiasis (VVC), and specific cases of infertility. These conditions are often associated with an imbalance in the vaginal microbiota, characterized by a decrease in beneficial Lactobacillus species and an overgrowth of pathogenic bacteria or fungi. The use of microbiota transplantation from healthy women has been suggested as a potential therapy to address imbalances in the vaginal microbiota, known as vaginal dysbiosis. Studies in rat models of vaginal dysbiosis have demonstrated that VMT can be therapeutically effective in reducing inflammation and increasing the presence of Lactobacilli, as well as relieving endometritis-like symptoms (inflammation of the lining of the uterus). The benefits of therapeutic VMT have also been demonstrated in patients with symptomatic, intractable, and recurrent bacterial vaginosis, and this has opened a new avenue for such future studies.
Bacterial vaginosis (BV) refers to a condition in which there is a disturbance in the normal microbial community of the vagina. As well previously documented, usually, the vagina is predominantly inhabited by Lactobacillus species. However, in BV presence, there is a shift in the vaginal microbiome, leading to the emergence of anaerobic bacteria. BV may be associated with a risk of upper genital tract infections, pregnancy complications, and susceptibility to sexually transmitted infections. Currently, there are many limited treatment options in patients with persistent or recurrent BV despite multiple attempts at antibiotic treatment. In addition, probiotic treatment of symptomatic patients with oral and/or vaginal administration of bacterial strains of Lactobacillus has yielded conflicting results, suggesting that the microbiome as a whole, rather than a single bacterial species, may be effective for severe BV.
An experience presentation
Lev-Sagie et al. performed the first exploratory study testing the VMT approach from healthy donors as a therapeutic alternative for patients suffering from symptomatic, intractable, and recurrent bacterial vaginosis (Clinicaltrials.gov NCT02236429). Four out of five VMT recipients experienced a significant improvement in both clinical symptoms and the composition and function of the dysbiotic vaginal microbiome, which persisted over an extended follow-up period, while one recipient experienced partial remission. The authors reported no significant side effects and no serious adverse events. While the lack of adverse outcomes is reassuring, the small study size and lack of a placebo arm make it difficult to interpret whether VMT provided an additional benefit over antibiotics alone. Additional clinical trials are currently ongoing with the goal of further evaluating whether VMT could serve as a viable option in symptomatic and intractable BV
Other Microbiota-Changing Strategies and Future Perspectives
In addition to FM, there are other strategies such as diet, and the administration of prebiotics, probiotics , synbiotics, and postbiotics. Recent research has documented that more than 50% of the diversity in the microbiome can be attributed to food, while host genetics only slightly influence the composition of the microbiota. In detail, a diet high in fiber promotes a significant rise in species producing SCFAs. A high-protein, high-fat, low-fiber diet, on the other hand, is linked to decreased biodiversity and an increase in species that could cause inflammation. A brief food change may cause a change in the gut’s population, but these modifications seem to be temporary. Diet may increase the success of FMT by fostering a favorable environment for the engraftment of donor microbiota and by exerting its own anti-inflammatory effects.
However, these natural approaches lack a direct-targeting action for gut microbiota shaping. For this purpose, researchers have developed the use of engineered bacterial (EB) strains to influence and manipulate the composition and behavior of the microbiota in a promising targeted manner. EB strains can be designed to produce specific metabolites or molecules that, interacting with the microbiota, can influence its composition or activity, showing beneficial effects on human health. EBs are rightly considered the next-generation microbiota therapeutics. In addition, they can be designed to express functions that address monogenic inborn errors of metabolism or to exert a tumor-killing activity.
However, although FMT is a valid therapeutic approach for certain diseases, it shows some procedural limitations. The preparation of fresh or frozen fecal suspensions requires the constant and periodic presence of healthy donors which must be negative in a series of serological and microbiological screening tests. Moreover, the stool samples have to be processed within six hours after collection for preserving anaerobes.
Furthermore, it is essential to know in detail the bacterial composition of the suspension to be infused into the recipient, in order to increase FMT efficacy and especially safety. For these reasons, the therapeutic efficacy of a synthetic bacterial preparation called “Bacterial Consortium” is now under development. This approach involves the isolation from healthy donors’ stool samples of several bacterial species normally present in the human intestinal microbiota, and thus the use of this “Bacterial Consortium”, composed by 13 microbial species, as a safe and valid alternative to donor stools. In conclusion, understanding the mechanisms by which the human microbiota can influence the progression of diseases, including gynecological disorders, can lead to the development of personalized approaches to shape the microbiota composition (and so its function), improving the symptoms and patients’ prognosis.
References
- Li, P.; Shuai, P.; Shen, S.; Zheng, H.; Sun, P.; Zhang, R.; Lan, S.; Lan, Z.; Jayawardana, T.; Yang, Y.; et al. Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: A systematic review and meta-analysis. BMC Med. ۲۰۲۳, ۲۱, ۳۰۲.
- Martinelli S, Nannini G, Cianchi F, Staderini F, Coratti F, Amedei A. Microbiota Transplant and Gynecological Disorders: The Bridge between Present and Future Treatments. Microorganisms. 2023 Sep 27; 11(10):2407.
Provided by: Dr. Nazila Kassaian